Pipeline
ABL501
HomePipelineABL501
-
- Pipeline
- ABL501
-
- Program Target
- PD-L1 x LAG-3
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development
(Phase 1)
- Summary
- ABL501 is a bispecific antibody capable of blocking the PD-L1 and LAG-3 immune checkpoint pathways, overcoming the current limitations of conventional PD-(L)1 therapy based around resistance to PD-L1 and low response rates. ABL501 demonstrates a higher efficacy over LAG-3 alone, PD-L1 alone, and the combination of LAG-3 and PD-L1, and shows superior performance compared to competing substances.
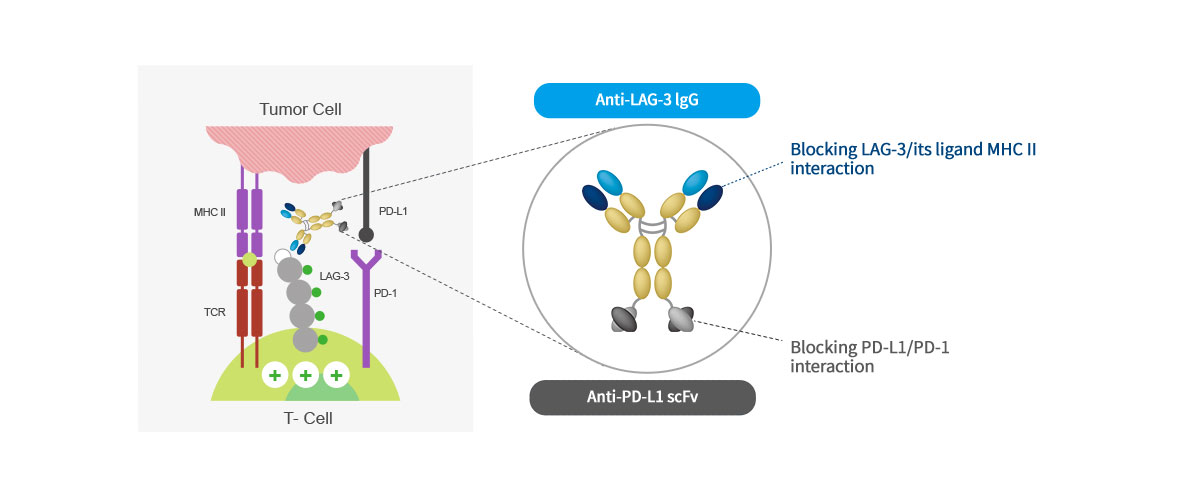
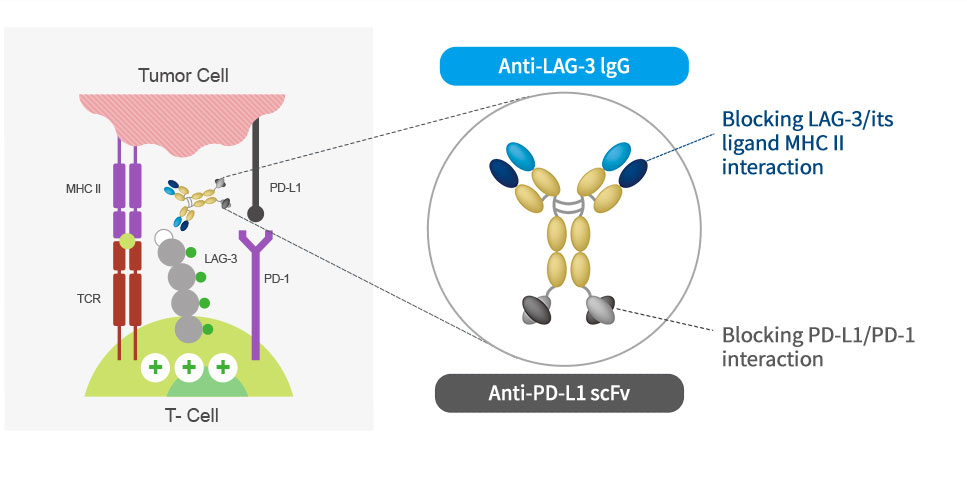
Mechanism of action
ABL501 is a bispecific antibody targeting LAG-3 and PD-L1 and blocks the interaction of LAG3-MHCII and PD-1-PD-L1,
releasing T cells from tumor-mediated suppression

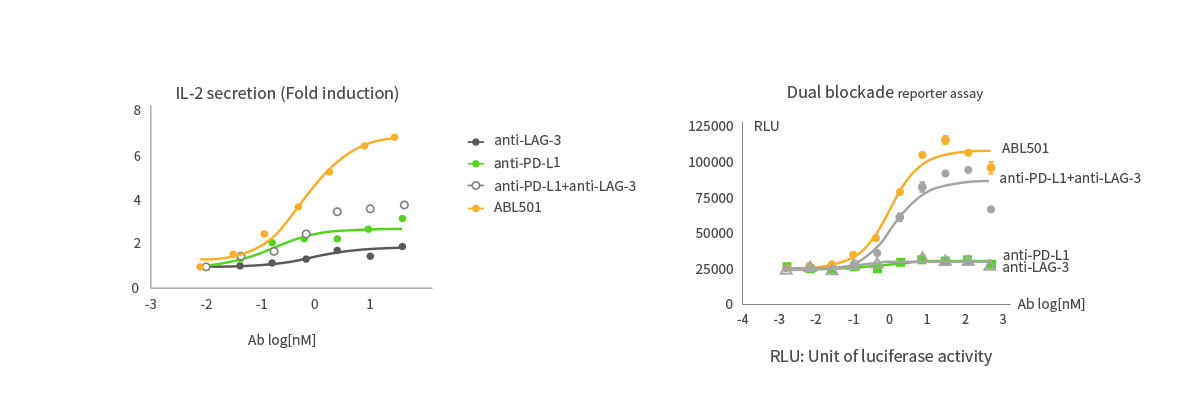
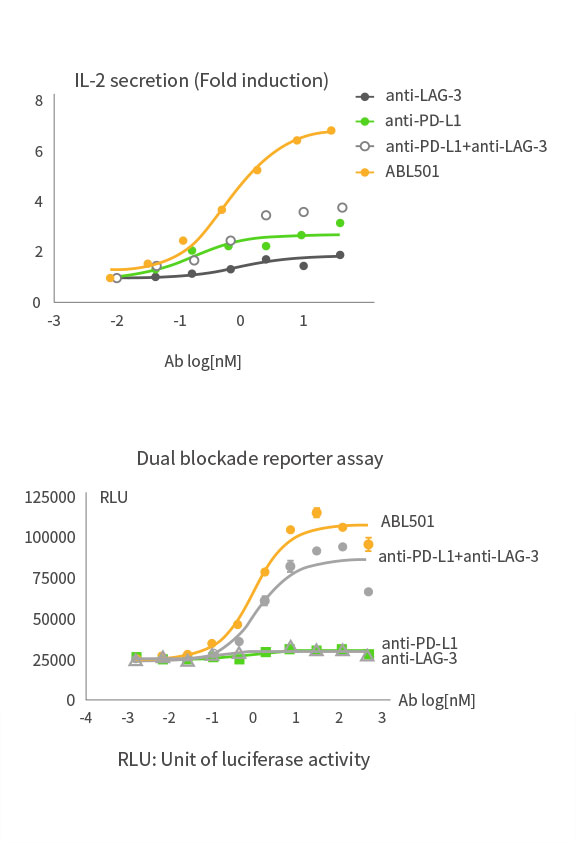
Higher T cell activation than combination of LAG-3 and PD-L1 antibodies
ABL501 induced superior T cell activation compared to the combination of LAG-3 and PD-L1 antibodies as indicated in PBMC-based IL-2 expression assays and dual blockade reporter assays
Strong tumor killing effect of ABL501 compared with Atezolizumab
ABL501 induced stronger cytotoxic activity on NSCLC cells in the presence of tumor-matched patient PBMCs
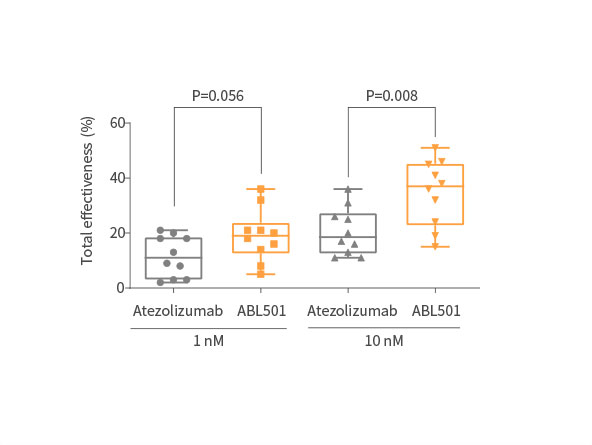
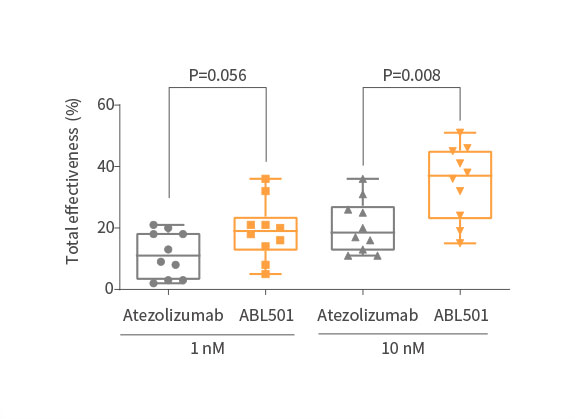
Potent inhibition on tumor progression
ABL501 exhibited dose-dependent tumor growth inhibition
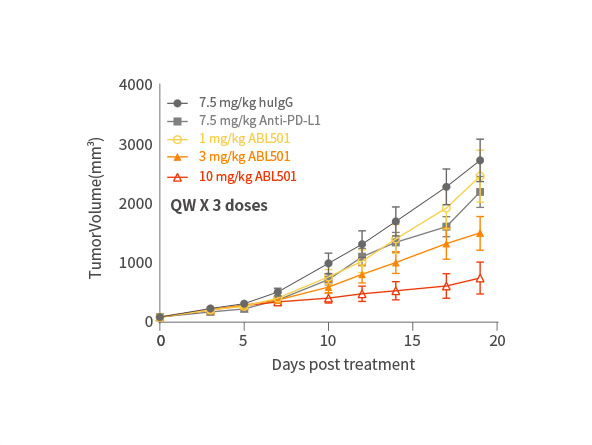
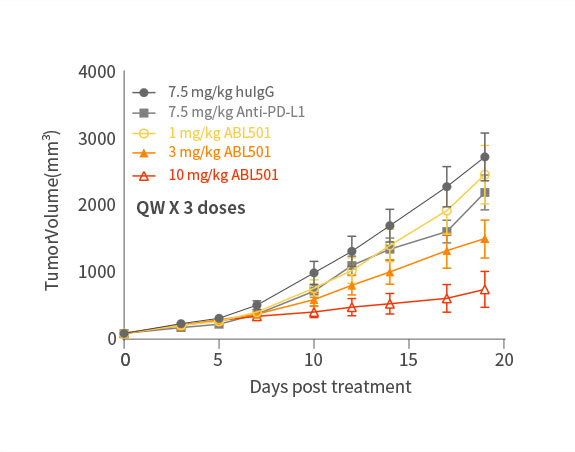
Phase 1 clinical study
Monotherapy dose escalation is ongoing with support of Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN21C0979000021, Republic of Korea).