Pipeline
ABL111
HomePipelineABL111
-
- Pipeline
- ABL111 (Givastomig)
-
- Program Target
- Claudin18.2x4-1BB
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development
(Phase 1)
- Summary
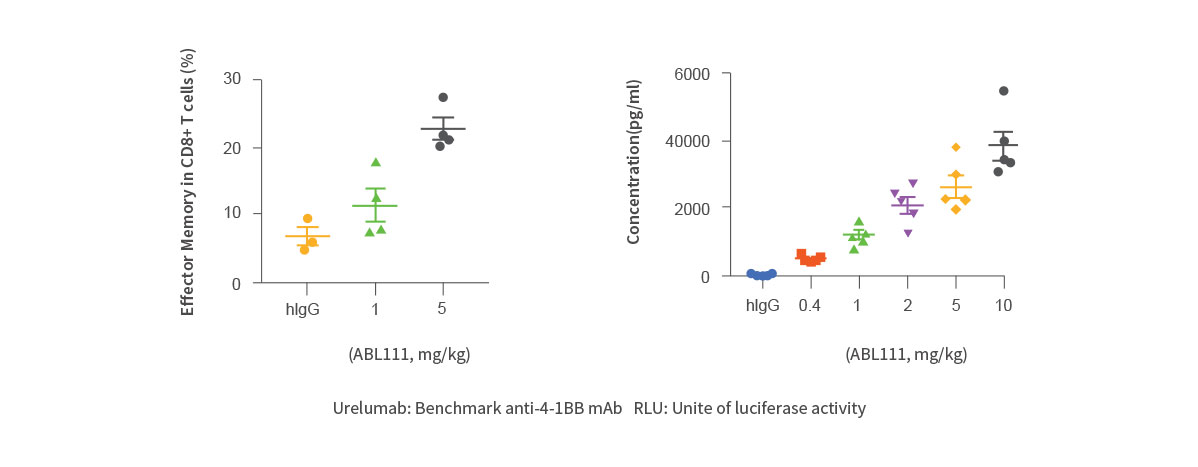
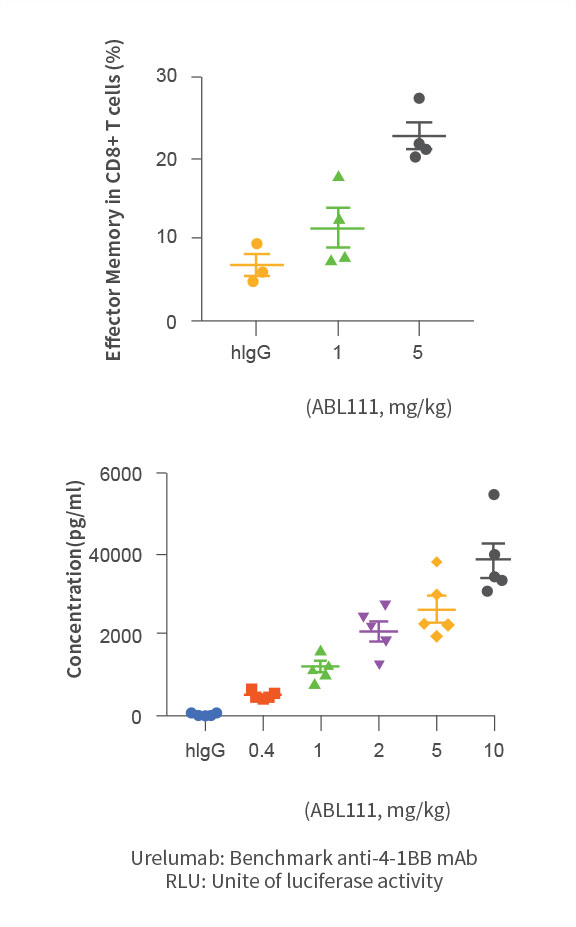
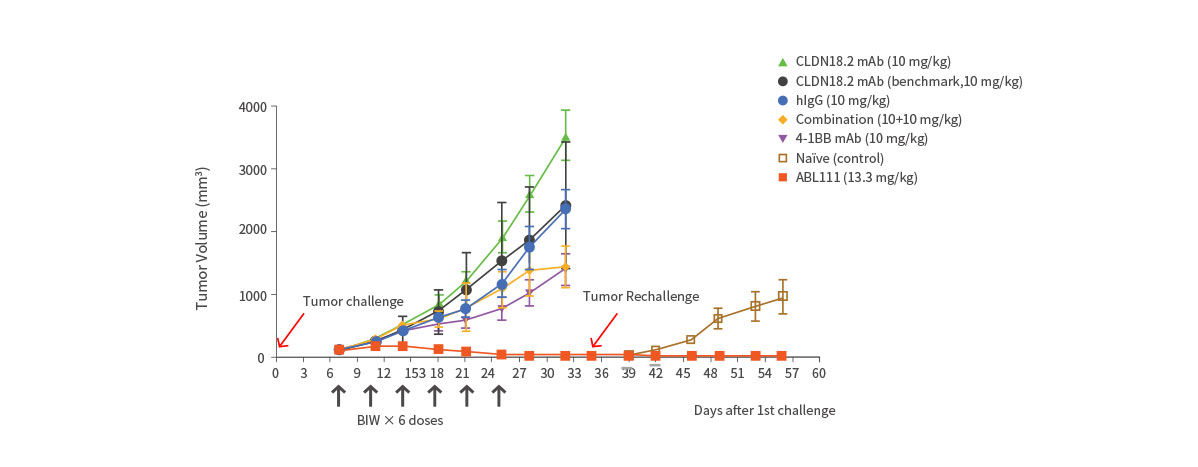
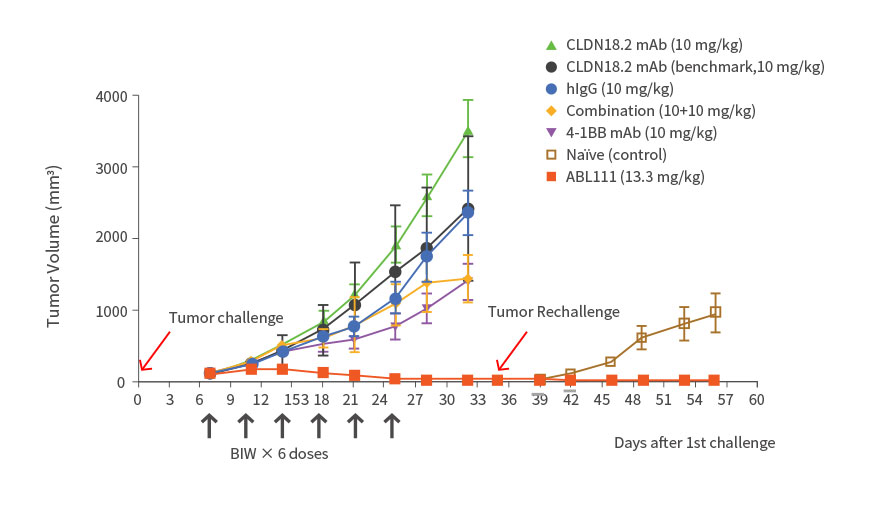
- ABL111 is a bispecific antibody combining Claudin18.2, a gastric- and pancreatic-specific cancer antigen with 4-1BB agonistic activity to supercharge T cells in a Claudin18.2-dependent manner, enhancing anti-tumor immunity while minimizing 4-1BB related toxicity. ABL111 is full length anti-Claudin18.2 mAb (Fc-silenced human IgG1) fused with scFv of anti-4-1BB engaging mAb. ABL111 possesses unique properties that 4-1BB activation signal is dependent on Claudin18.2 expression. ABL111 shows superior anti-tumor activity in an animal model system than Claudin18.2 alone, 4-1BB alone or combination of both with immunological memory resistant to rechallenge with same tumors.
Structure and Mechanism of Action
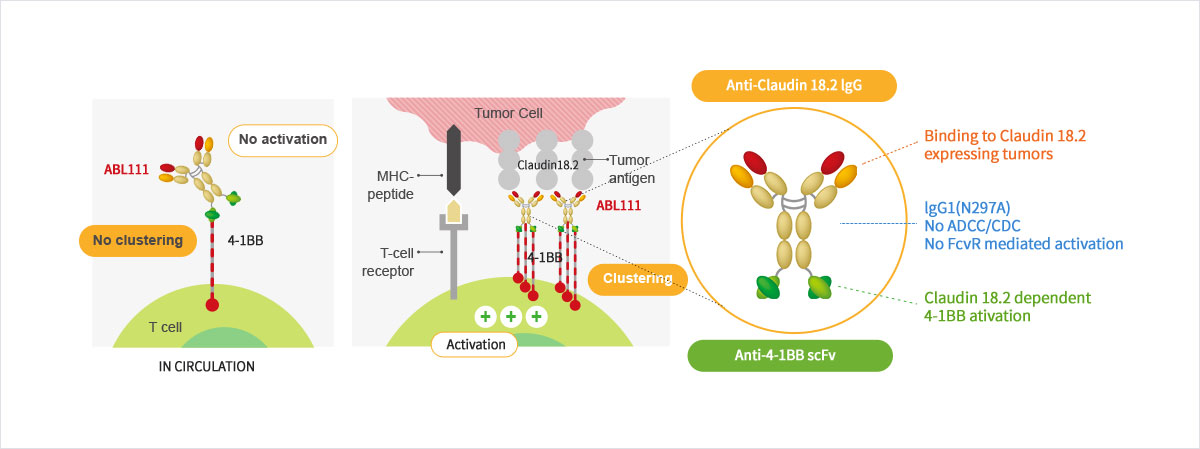

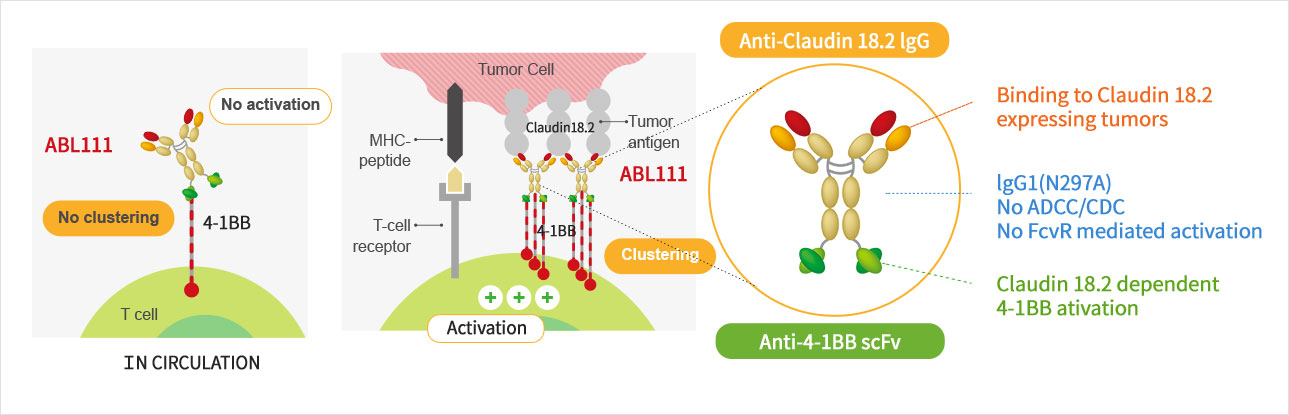
Claudin18.2 Dependent 4-1BB Activation
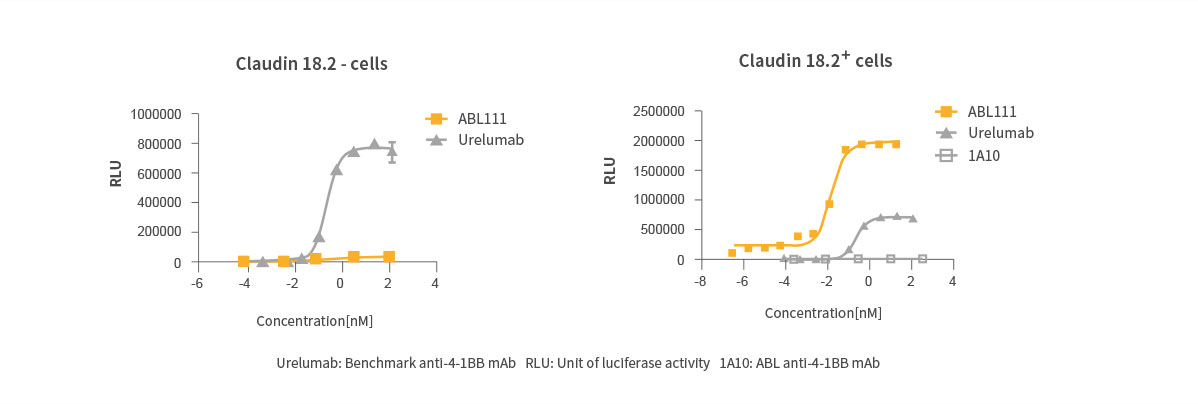

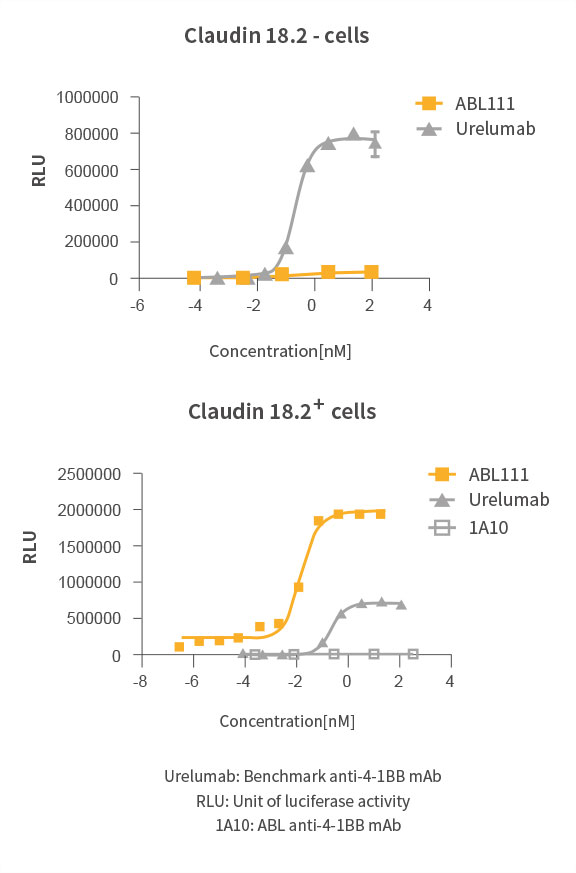
Superior Anti-Tumor Effect with Immunological Memory

Increase of effector memory CD8+T cells and soluble 4-1BB