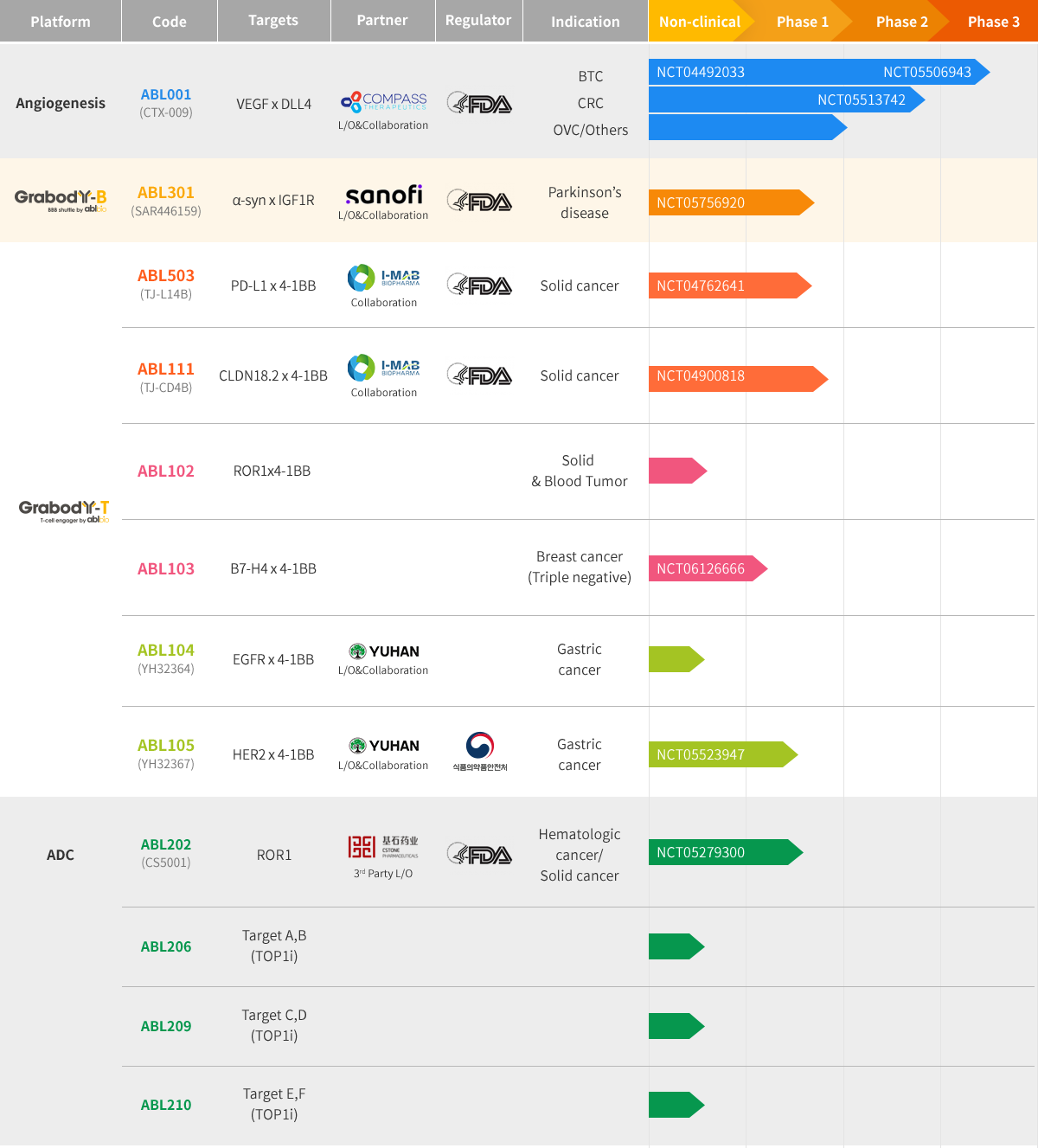
Pipeline
Overview
HomePipelineOverview
ABL001
-
- Pipeline
- ABL001
-
- Program Target
- VEGF x DLL4
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development
(Phase 1a/1b)
- Summary
- Better anti-cancer efficacy of ABL001 at in vitro/in vivo studies are confirmed with cancer patients in clinical trial (phase 1a/1b).
ABL001 can be developed as the next generation of anti-angiogenic therapy.
ABL105
-
- Pipeline
- ABL105
-
- Program Target
- HER2x4-1BB
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development (Phase 1)
- Summary
- ABL105 is a bispecific antibody that simultaneously HER2 and 4-1BB, crosslinking and activating 4-1BB signaling only in the presence of HER2 expressing tumor cells. Additionally, ABL105 blocks HER2 signaling and induces NK cell-mediated cytotoxicity, leading to stronger anti-tumor activity. ABL105 significantly inhibits tumor growth and protects mice from tumor recurrence by inducing tumor-specific immunological memory.
ABL111
-
- Pipeline
- ABL111 (Givastomig)
-
- Program Target
- Claudin18.2x4-1BB
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development
(Phase 1)
- Summary
- ABL111 is a bispecific antibody that targets Claudin18.2, a tumor antigen overexpressed in gastric- and pancreatic-specific cancer, and 4-1BB, a co-stimulatory receptor with the ability to induce potent anti-tumor activity. To minimize the usual toxicity associated with monospecific 4-1BB based treatments, ABL111 only activates the 4-1BB pathway when engaged with Claudin 18.2, thus triggering T cell activation and enhancing anti-tumor immunity while minimizing toxicity. ABL111 shows superior anti-tumor activity in an animal model system compared to Claudin18.2 alone, 4-1BB alone, and the combination of both Claudin18.2 and 4-1BB, with immunological memory resistant to any possible recurrence of the cancer cells.
ABL301
-
- Pipeline
- ABL301SAR446159
-
- Program Target
- SNCA x IGF1R
-
- Disease Indication
- Parkinson’s Disease
-
- Development Stage
- IND enabling study
- Summary
- The propagation of aggregated forms of alpha-synuclein (α-synuclein, SNCA) appears to be critical for the etiology of Parkinson’s disease and multiple system atrophy. ABL301 selectively targets aggregated forms of α-synuclein as a potential disease target, avoiding its normal, monomeric form. Since poor delivery of antibodies pastsing the blood-brain-barrier is thought to be one of the major obstacles for CNS-related drug development, ABL301 has a shuttle antibody(IGF1R antibodyL Grabody B) that allows for improved delivery of antibody therapeutics into the brain. This dual mechanism will enable antibody therapeutics to more efficiently reach their targets, leading to better therapeutic efficacy compared to conventional monoclonal antibodies.
ABL501
-
- Pipeline
- ABL501
-
- Program Target
- PD-L1 x LAG-3
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development
(Phase 1)
- Summary
- ABL501 is a bispecific antibody capable of blocking the PD-L1 and LAG-3 immune checkpoint pathways, overcoming the current limitations of conventional PD-(L)1 therapy based around resistance to PD-L1 and low response rates. ABL501 demonstrates a higher efficacy over LAG-3 alone, PD-L1 alone, and the combination of LAG-3 and PD-L1, and shows superior performance compared to competing substances.
ABL503
-
- Pipeline
- ABL503 (Ragistomig)
-
- Program Target
- PD-L1 x 4-1BB
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- Clinical Development
(Phase 1)
- Summary
- ABL503 is a bispecific antibody that combines the capabilities of PD-L1 checkpoint pathway inhibiton with 4-1BB agonistic activity to overcome the specific limitations of PD-(L)1 immunotherapy and 4-1BB related toxicity. ABL503 is uniquely designed to only induce 4-1BB signalling when simultaneously binded to the PD-L1 tumor antigen on cancer cells, limiting the toxicity characteristic of traditional 4-1BB based treatment while augmenting T-cell activation. ABL503 shows superior anti-tumor activity in an animal model system compared to PD-L1 alone, 4-1BB alone, and the combination of PD-L1 and 4-1BB, with immunological memory resistant to any possible recurrence of the tumor.
ABL101
-
- Pipeline
- ABL101
-
- Program Target
- BCMAx4-1BB
-
- Disease Indication
- Hematologic Cancer
-
- Development Stage
- IND enabling study
- Summary
- A novel T-cell engaging bispecific antibody, ABL101 demonstrates a robust anti-tumor effect via BCMA mediated 4-1BB activation in tumor microenvironments (TME). ABL101 only activates 4-1BB signaling pathways in the presence of BCMA expressing cancer cells, minimizing the probability of hepatotoxicity in healthy tissues. ABL101 inhibits tumor growth and, by retaining immunological memory, induces a prolonged anti-tumor effect.
ABL103
-
- Pipeline
- ABL103
-
- Program Target
- B7-H4x4-1BB
-
- Disease Indication
- Solid Cancer
-
- Development Stage
- IND enabling study
- Summary
- A novel T-cell engaging bispecific antibody, ABL103 demonstrates efficacious anti-tumor activity via B7-H4 mediated 4-1BB activation in tumor microenvironments (TME), only activating 4-1BB signaling pathways when in the presence of B7-H4 expressing cells. ABL103 strongly inhibits tumor growth and induces immunological memory to create prolonged anti-tumor protection.
ABL102
-
- Pipeline
- ABL102
-
- Program Target
- ROR1x4-1BB
-
- Disease Indication
- Solid & Blood Tumor
-
- Development Stage
- IND enabling study
- Summary
- ABL102 is a bispecific antibody that simultaneously targets ROR1 and 4-1BB, activating 4-1BB signaling only in the presence of ROR1 expressing tumor cells. By having intact Fc, ABL102 activates T cells via FcgRI mediated 4-1BB clustering. Additionally, ABL102 induces intra-tumoral Treg depletion potentially via an ADCC activity, resulting in strong tumor growth inbihition. ABL102 significantly inhibits tumor growth and protects mice from tumor recurrence inducing tumor-specific immunological memory.
ABL104
-
- Pipeline
- ABL104
-
- Program Target
- EGFRx4-1BB
-
- Disease Indication
- Solid Tumor
-
- Development Stage
- IND enabling study
- Summary
- ABL104 is a bispecific antibody that simultaneously targets EGFR and 4-1BB, crosslinking and activating 4-1BB signaling only in the presence of EGFR-expressing tumor cells. Additionally, ABL104 induces stronger antitumor activity by blocking EGFR signaling. ABL104 significantly inhibits tumor growth and portects mice from tumor recurrence by inducing tumor-specific immunological memory.
ABL602
-
- Pipeline
- ABL602
-
- Program Target
- CLL1xCD3
-
- Disease Indication
- Blood Cancer
-
- Development Stage
- IND enabling study
- Summary
- ABL602 is a head-to-tail 2+1 bispecific T cell engager targeting CLL1. ABL602 binds weakly to CD3 in normal tissue, but its binding is greatly potentiated in tumor microenvironments (TME). ABL602 exhibits anti-tumor activity via CLL1 mediated T cell activation and does not induces cytokines in the absence of CLL1. ABL602 is expected to be a safer and more effective therapy for AML.